Essais CLiniques Accessibles Interconnectés pour la Recherche ouverts à l'Ecosystème
0.1.0 - trial-use
Essais CLiniques Accessibles Interconnectés pour la Recherche ouverts à l'Ecosystème
0.1.0 - trial-use
This page is part of the Implementation Guide FHIR pour le projet ECLAIRE, base de données qui recense les essais cliniques en France (v0.1.0: Release) based on FHIR R4. This is the current published version in its permanent home (it will always be available at this URL). For a full list of available versions, see the Directory of published versions 
| Official URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-researchstudy | Version: 0.1.0 | |||
| Draft as of 2023-07-28 | Computable Name: ECLAIREResearchStudy | |||
Profil de ResearchStudy pour le projet ECLAIRE
Usage:
Description of Profiles, Differentials, Snapshots and how the different presentations work.
This structure is derived from ResearchStudy
Summary
Mandatory: 0 element (2 nested mandatory elements)
Must-Support: 20 elements
Extensions
This structure refers to these extensions:
Slices
This structure defines the following Slices:
This structure is derived from ResearchStudy
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | ResearchStudy | Investigation to increase healthcare-related patient-independent knowledge | |
  | S | 0..1 | date | Date de dernière modification substancielle URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-review-date |
  | S | 0..1 | string | Précisions sur le sujet / Health Condition(s) or Problem(s) Studied URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-condition-details |
  | S | 0..1 | string | Domaine thérapeutique concerné URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-therapeutic-area |
  | S | 0..1 | Period | Période prévisionnelle de recrutement URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-recruitment-period |
  | S | 0..* | Reference(Organization) | Promoteur(s) secondaire(s) / Secondary Sponsor(s) URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-secondary-sponsor |
  | S | 0..* | (Complex) | autres titres et acronyme / Additional names for the study URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-label |
  | 0..* | Identifier | Business Identifier for study Slice: Unordered, Open by value:use | |
  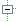 | S | 0..1 | Identifier | Identifiant primaire de l'essai clinique / Primary Registry and Trial Identifying Number |
  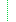  | 1..1 | code | usual | official | temp | secondary | old (If known) Required Pattern: official | |
  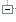 | S | 0..* | Identifier | identifiants secondaires / Secondary Identifying Numbers (e.g., protocol number) if available. Also include other trial registries that have issued an identifying number to this trial. There is no limit on the number of Secondary identifying numbers that can be provided. |
    | 1..1 | code | usual | official | temp | secondary | old (If known) Required Pattern: secondary | |
  | S | 0..1 | string | Nom scientifique de l'étude / Scientific Title |
  | S | 1..1 | code | Statut de l'essai / Study Status |
  | S | 0..1 | CodeableConcept | Phase de l'essai / Study type : phase Binding: Value Set type de phase Eclaire de l'essai (extensible) |
  | S | 0..* | CodeableConcept | Type d'essai / Study type : type of study |
  | S | 0..* | CodeableConcept | Sujet concerné / Problem(s) Studied exemple code MedDRA |
  | S | 0..* | ContactDetail | Contact (Contact for public / scientific queries) |
   | S | 0..1 | CodeableConcept | type de contact : Public ou Scientific URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-contact-type Binding: Type Contact Value Set (required) |
  | S | 0..* | CodeableConcept | Pays de recrutement / Countries of Recruitment |
  | S | 0..1 | markdown | Résumé de l'essai / Summary Results |
  | S | 0..* | Reference(Group) | Critères d'inclusion et d'exclusion / Inclusion & exclusion criteria |
  | S | 0..1 | Reference(Organization) | Promoteur / primary Sponsor |
  | S | 0..* | Reference(Location) | Lieux / Countries of Recruitment |
 Documentation for this format Documentation for this format | ||||
| Name | Flags | Card. | Type | Description & Constraints | ||||
|---|---|---|---|---|---|---|---|---|
 | 0..* | ResearchStudy | Investigation to increase healthcare-related patient-independent knowledge | |||||
  | Σ | 0..1 | id | Logical id of this artifact | ||||
  | ΣN | 0..1 | Meta | Metadata about the resource | ||||
  | ?!ΣN | 0..1 | uri | A set of rules under which this content was created | ||||
  | N | 0..1 | code | Language of the resource content Binding: CommonLanguages (preferred): A human language.
| ||||
  | N | 0..1 | Narrative | Text summary of the resource, for human interpretation | ||||
  | 0..* | Resource | Contained, inline Resources | |||||
  | N | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url | ||||
  | S | 0..1 | date | Date de dernière modification substancielle URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-review-date | ||||
  | S | 0..1 | string | Précisions sur le sujet / Health Condition(s) or Problem(s) Studied URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-condition-details | ||||
  | S | 0..1 | string | Domaine thérapeutique concerné URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-therapeutic-area | ||||
  | S | 0..1 | Period | Période prévisionnelle de recrutement URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-recruitment-period | ||||
  | S | 0..* | Reference(Organization) | Promoteur(s) secondaire(s) / Secondary Sponsor(s) URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-secondary-sponsor | ||||
  | S | 0..* | (Complex) | autres titres et acronyme / Additional names for the study URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-label | ||||
  | ?!N | 0..* | Extension | Extensions that cannot be ignored Slice: Unordered, Open by value:url | ||||
  | Σ | 0..* | Identifier | Business Identifier for study Slice: Unordered, Open by value:use | ||||
   | SΣ | 0..1 | Identifier | Identifiant primaire de l'essai clinique / Primary Registry and Trial Identifying Number | ||||
    | 0..1 | string | Unique id for inter-element referencing | |||||
    | N | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url | ||||
    | ?!Σ | 1..1 | code | usual | official | temp | secondary | old (If known) Binding: IdentifierUse (required): Identifies the purpose for this identifier, if known . Required Pattern: official | ||||
    | ΣN | 0..1 | CodeableConcept | Description of identifier Binding: Identifier Type Codes (extensible): A coded type for an identifier that can be used to determine which identifier to use for a specific purpose. | ||||
    | ΣN | 0..1 | uri | The namespace for the identifier value Example General: http://www.acme.com/identifiers/patient | ||||
    | ΣN | 0..1 | string | The value that is unique Example General: 123456 | ||||
    | ΣCN | 0..1 | Period | Time period when id is/was valid for use | ||||
    | ΣCN | 0..1 | Reference(Organization) | Organization that issued id (may be just text) | ||||
   | SΣ | 0..* | Identifier | identifiants secondaires / Secondary Identifying Numbers (e.g., protocol number) if available. Also include other trial registries that have issued an identifying number to this trial. There is no limit on the number of Secondary identifying numbers that can be provided. | ||||
    | 0..1 | string | Unique id for inter-element referencing | |||||
    | N | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url | ||||
    | ?!Σ | 1..1 | code | usual | official | temp | secondary | old (If known) Binding: IdentifierUse (required): Identifies the purpose for this identifier, if known . Required Pattern: secondary | ||||
    | ΣN | 0..1 | CodeableConcept | Description of identifier Binding: Identifier Type Codes (extensible): A coded type for an identifier that can be used to determine which identifier to use for a specific purpose. | ||||
    | ΣN | 0..1 | uri | The namespace for the identifier value Example General: http://www.acme.com/identifiers/patient | ||||
    | ΣN | 0..1 | string | The value that is unique Example General: 123456 | ||||
    | ΣCN | 0..1 | Period | Time period when id is/was valid for use | ||||
    | ΣCN | 0..1 | Reference(Organization) | Organization that issued id (may be just text) | ||||
  | SΣ | 0..1 | string | Nom scientifique de l'étude / Scientific Title | ||||
  | ΣCN | 0..* | Reference(PlanDefinition) | Steps followed in executing study | ||||
  | ΣCN | 0..* | Reference(ResearchStudy) | Part of larger study | ||||
  | ?!SΣ | 1..1 | code | Statut de l'essai / Study Status Binding: ResearchStudyStatus (required): Codes that convey the current status of the research study. | ||||
  | ΣN | 0..1 | CodeableConcept | treatment | prevention | diagnostic | supportive-care | screening | health-services-research | basic-science | device-feasibility Binding: ResearchStudyPrimaryPurposeType (extensible): Codes for the main intent of the study. | ||||
  | SΣ | 0..1 | CodeableConcept | Phase de l'essai / Study type : phase Binding: Value Set type de phase Eclaire de l'essai (extensible) | ||||
  | SΣ | 0..* | CodeableConcept | Type d'essai / Study type : type of study Binding: (unbound) (example): Codes that describe the type of research study. E.g. Study phase, Interventional/Observational, blinding type, etc. | ||||
  | ΣN | 0..* | CodeableConcept | Drugs, devices, etc. under study Binding: (unbound) (example): Codes for medications, devices and other interventions. | ||||
  | SΣ | 0..* | CodeableConcept | Sujet concerné / Problem(s) Studied exemple code MedDRA Binding: Condition/Problem/DiagnosisCodes (example): Identification of the condition or diagnosis. | ||||
  | SΣ | 0..* | ContactDetail | Contact (Contact for public / scientific queries) | ||||
   | 0..1 | string | Unique id for inter-element referencing | |||||
   | N | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url | ||||
   | S | 0..1 | CodeableConcept | type de contact : Public ou Scientific URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-contact-type Binding: Type Contact Value Set (required) | ||||
   | ΣN | 0..1 | string | Name of an individual to contact | ||||
   | ΣCN | 0..* | ContactPoint | Contact details for individual or organization | ||||
  | TU | 0..* | RelatedArtifact | References and dependencies | ||||
  | ΣN | 0..* | CodeableConcept | Used to search for the study Binding: (unbound) (example): Words associated with the study that may be useful in discovery. | ||||
  | SΣ | 0..* | CodeableConcept | Pays de recrutement / Countries of Recruitment Binding: Jurisdiction ValueSet (extensible): Countries and regions within which this artifact is targeted for use. | ||||
  | S | 0..1 | markdown | Résumé de l'essai / Summary Results | ||||
  | SΣC | 0..* | Reference(Group) | Critères d'inclusion et d'exclusion / Inclusion & exclusion criteria | ||||
  | ΣCN | 0..1 | Period | When the study began and ended | ||||
  | SΣC | 0..1 | Reference(Organization) | Promoteur / primary Sponsor | ||||
  | ΣCN | 0..1 | Reference(Practitioner | PractitionerRole) | Researcher who oversees multiple aspects of the study | ||||
  | SΣC | 0..* | Reference(Location) | Lieux / Countries of Recruitment | ||||
  | ΣN | 0..1 | CodeableConcept | accrual-goal-met | closed-due-to-toxicity | closed-due-to-lack-of-study-progress | temporarily-closed-per-study-design Binding: ResearchStudyReasonStopped (example): Codes for why the study ended prematurely. | ||||
  | N | 0..* | Annotation | Comments made about the study | ||||
  | N | 0..* | BackboneElement | Defined path through the study for a subject | ||||
   | 0..1 | string | Unique id for inter-element referencing | |||||
   | N | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url | ||||
   | ?!ΣN | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ||||
   | N | 1..1 | string | Label for study arm | ||||
   | N | 0..1 | CodeableConcept | Categorization of study arm | ||||
   | N | 0..1 | string | Short explanation of study path | ||||
  | N | 0..* | BackboneElement | A goal for the study | ||||
   | 0..1 | string | Unique id for inter-element referencing | |||||
   | N | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url | ||||
   | ?!ΣN | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ||||
   | N | 0..1 | string | Label for the objective | ||||
   | N | 0..1 | CodeableConcept | primary | secondary | exploratory Binding: ResearchStudyObjectiveType (preferred): Codes for the kind of study objective. | ||||
 Documentation for this format Documentation for this format | ||||||||
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | ResearchStudy | Investigation to increase healthcare-related patient-independent knowledge | |
  | 0..1 | date | Date de dernière modification substancielle URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-review-date | |
  | 0..1 | string | Précisions sur le sujet / Health Condition(s) or Problem(s) Studied URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-condition-details | |
  | 0..1 | string | Domaine thérapeutique concerné URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-therapeutic-area | |
  | 0..1 | Period | Période prévisionnelle de recrutement URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-recruitment-period | |
  | 0..* | Reference(Organization) | Promoteur(s) secondaire(s) / Secondary Sponsor(s) URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-secondary-sponsor | |
  | 0..* | (Complex) | autres titres et acronyme / Additional names for the study URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-label | |
  | Σ | 0..1 | Identifier | Identifiant primaire de l'essai clinique / Primary Registry and Trial Identifying Number |
  | Σ | 0..* | Identifier | identifiants secondaires / Secondary Identifying Numbers (e.g., protocol number) if available. Also include other trial registries that have issued an identifying number to this trial. There is no limit on the number of Secondary identifying numbers that can be provided. |
  | Σ | 0..1 | string | Nom scientifique de l'étude / Scientific Title |
  | ?!Σ | 1..1 | code | Statut de l'essai / Study Status Binding: ResearchStudyStatus (required): Codes that convey the current status of the research study. |
  | Σ | 0..1 | CodeableConcept | Phase de l'essai / Study type : phase Binding: Value Set type de phase Eclaire de l'essai (extensible) |
  | Σ | 0..* | CodeableConcept | Type d'essai / Study type : type of study Binding: (unbound) (example): Codes that describe the type of research study. E.g. Study phase, Interventional/Observational, blinding type, etc. |
  | Σ | 0..* | CodeableConcept | Sujet concerné / Problem(s) Studied exemple code MedDRA Binding: Condition/Problem/DiagnosisCodes (example): Identification of the condition or diagnosis. |
  | Σ | 0..* | ContactDetail | Contact (Contact for public / scientific queries) |
   | 0..1 | CodeableConcept | type de contact : Public ou Scientific URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-contact-type Binding: Type Contact Value Set (required) | |
  | Σ | 0..* | CodeableConcept | Pays de recrutement / Countries of Recruitment Binding: Jurisdiction ValueSet (extensible): Countries and regions within which this artifact is targeted for use. |
  | 0..1 | markdown | Résumé de l'essai / Summary Results | |
  | ΣC | 0..* | Reference(Group) | Critères d'inclusion et d'exclusion / Inclusion & exclusion criteria |
  | ΣC | 0..1 | Reference(Organization) | Promoteur / primary Sponsor |
  | ΣC | 0..* | Reference(Location) | Lieux / Countries of Recruitment |
 Documentation for this format Documentation for this format | ||||
This structure is derived from ResearchStudy
Summary
Mandatory: 0 element (2 nested mandatory elements)
Must-Support: 20 elements
Extensions
This structure refers to these extensions:
Slices
This structure defines the following Slices:
Differential View
This structure is derived from ResearchStudy
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | ResearchStudy | Investigation to increase healthcare-related patient-independent knowledge | |
  | S | 0..1 | date | Date de dernière modification substancielle URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-review-date |
  | S | 0..1 | string | Précisions sur le sujet / Health Condition(s) or Problem(s) Studied URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-condition-details |
  | S | 0..1 | string | Domaine thérapeutique concerné URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-therapeutic-area |
  | S | 0..1 | Period | Période prévisionnelle de recrutement URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-recruitment-period |
  | S | 0..* | Reference(Organization) | Promoteur(s) secondaire(s) / Secondary Sponsor(s) URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-secondary-sponsor |
  | S | 0..* | (Complex) | autres titres et acronyme / Additional names for the study URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-label |
  | 0..* | Identifier | Business Identifier for study Slice: Unordered, Open by value:use | |
   | S | 0..1 | Identifier | Identifiant primaire de l'essai clinique / Primary Registry and Trial Identifying Number |
    | 1..1 | code | usual | official | temp | secondary | old (If known) Required Pattern: official | |
   | S | 0..* | Identifier | identifiants secondaires / Secondary Identifying Numbers (e.g., protocol number) if available. Also include other trial registries that have issued an identifying number to this trial. There is no limit on the number of Secondary identifying numbers that can be provided. |
    | 1..1 | code | usual | official | temp | secondary | old (If known) Required Pattern: secondary | |
  | S | 0..1 | string | Nom scientifique de l'étude / Scientific Title |
  | S | 1..1 | code | Statut de l'essai / Study Status |
  | S | 0..1 | CodeableConcept | Phase de l'essai / Study type : phase Binding: Value Set type de phase Eclaire de l'essai (extensible) |
  | S | 0..* | CodeableConcept | Type d'essai / Study type : type of study |
  | S | 0..* | CodeableConcept | Sujet concerné / Problem(s) Studied exemple code MedDRA |
  | S | 0..* | ContactDetail | Contact (Contact for public / scientific queries) |
   | S | 0..1 | CodeableConcept | type de contact : Public ou Scientific URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-contact-type Binding: Type Contact Value Set (required) |
  | S | 0..* | CodeableConcept | Pays de recrutement / Countries of Recruitment |
  | S | 0..1 | markdown | Résumé de l'essai / Summary Results |
  | S | 0..* | Reference(Group) | Critères d'inclusion et d'exclusion / Inclusion & exclusion criteria |
  | S | 0..1 | Reference(Organization) | Promoteur / primary Sponsor |
  | S | 0..* | Reference(Location) | Lieux / Countries of Recruitment |
 Documentation for this format Documentation for this format | ||||
Snapshot View
| Name | Flags | Card. | Type | Description & Constraints | ||||
|---|---|---|---|---|---|---|---|---|
 | 0..* | ResearchStudy | Investigation to increase healthcare-related patient-independent knowledge | |||||
  | Σ | 0..1 | id | Logical id of this artifact | ||||
  | ΣN | 0..1 | Meta | Metadata about the resource | ||||
  | ?!ΣN | 0..1 | uri | A set of rules under which this content was created | ||||
  | N | 0..1 | code | Language of the resource content Binding: CommonLanguages (preferred): A human language.
| ||||
  | N | 0..1 | Narrative | Text summary of the resource, for human interpretation | ||||
  | 0..* | Resource | Contained, inline Resources | |||||
  | N | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url | ||||
  | S | 0..1 | date | Date de dernière modification substancielle URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-review-date | ||||
  | S | 0..1 | string | Précisions sur le sujet / Health Condition(s) or Problem(s) Studied URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-condition-details | ||||
  | S | 0..1 | string | Domaine thérapeutique concerné URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-therapeutic-area | ||||
  | S | 0..1 | Period | Période prévisionnelle de recrutement URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-recruitment-period | ||||
  | S | 0..* | Reference(Organization) | Promoteur(s) secondaire(s) / Secondary Sponsor(s) URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-secondary-sponsor | ||||
  | S | 0..* | (Complex) | autres titres et acronyme / Additional names for the study URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-label | ||||
  | ?!N | 0..* | Extension | Extensions that cannot be ignored Slice: Unordered, Open by value:url | ||||
  | Σ | 0..* | Identifier | Business Identifier for study Slice: Unordered, Open by value:use | ||||
   | SΣ | 0..1 | Identifier | Identifiant primaire de l'essai clinique / Primary Registry and Trial Identifying Number | ||||
    | 0..1 | string | Unique id for inter-element referencing | |||||
    | N | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url | ||||
    | ?!Σ | 1..1 | code | usual | official | temp | secondary | old (If known) Binding: IdentifierUse (required): Identifies the purpose for this identifier, if known . Required Pattern: official | ||||
    | ΣN | 0..1 | CodeableConcept | Description of identifier Binding: Identifier Type Codes (extensible): A coded type for an identifier that can be used to determine which identifier to use for a specific purpose. | ||||
    | ΣN | 0..1 | uri | The namespace for the identifier value Example General: http://www.acme.com/identifiers/patient | ||||
    | ΣN | 0..1 | string | The value that is unique Example General: 123456 | ||||
    | ΣCN | 0..1 | Period | Time period when id is/was valid for use | ||||
    | ΣCN | 0..1 | Reference(Organization) | Organization that issued id (may be just text) | ||||
   | SΣ | 0..* | Identifier | identifiants secondaires / Secondary Identifying Numbers (e.g., protocol number) if available. Also include other trial registries that have issued an identifying number to this trial. There is no limit on the number of Secondary identifying numbers that can be provided. | ||||
    | 0..1 | string | Unique id for inter-element referencing | |||||
    | N | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url | ||||
    | ?!Σ | 1..1 | code | usual | official | temp | secondary | old (If known) Binding: IdentifierUse (required): Identifies the purpose for this identifier, if known . Required Pattern: secondary | ||||
    | ΣN | 0..1 | CodeableConcept | Description of identifier Binding: Identifier Type Codes (extensible): A coded type for an identifier that can be used to determine which identifier to use for a specific purpose. | ||||
    | ΣN | 0..1 | uri | The namespace for the identifier value Example General: http://www.acme.com/identifiers/patient | ||||
    | ΣN | 0..1 | string | The value that is unique Example General: 123456 | ||||
    | ΣCN | 0..1 | Period | Time period when id is/was valid for use | ||||
    | ΣCN | 0..1 | Reference(Organization) | Organization that issued id (may be just text) | ||||
  | SΣ | 0..1 | string | Nom scientifique de l'étude / Scientific Title | ||||
  | ΣCN | 0..* | Reference(PlanDefinition) | Steps followed in executing study | ||||
  | ΣCN | 0..* | Reference(ResearchStudy) | Part of larger study | ||||
  | ?!SΣ | 1..1 | code | Statut de l'essai / Study Status Binding: ResearchStudyStatus (required): Codes that convey the current status of the research study. | ||||
  | ΣN | 0..1 | CodeableConcept | treatment | prevention | diagnostic | supportive-care | screening | health-services-research | basic-science | device-feasibility Binding: ResearchStudyPrimaryPurposeType (extensible): Codes for the main intent of the study. | ||||
  | SΣ | 0..1 | CodeableConcept | Phase de l'essai / Study type : phase Binding: Value Set type de phase Eclaire de l'essai (extensible) | ||||
  | SΣ | 0..* | CodeableConcept | Type d'essai / Study type : type of study Binding: (unbound) (example): Codes that describe the type of research study. E.g. Study phase, Interventional/Observational, blinding type, etc. | ||||
  | ΣN | 0..* | CodeableConcept | Drugs, devices, etc. under study Binding: (unbound) (example): Codes for medications, devices and other interventions. | ||||
  | SΣ | 0..* | CodeableConcept | Sujet concerné / Problem(s) Studied exemple code MedDRA Binding: Condition/Problem/DiagnosisCodes (example): Identification of the condition or diagnosis. | ||||
  | SΣ | 0..* | ContactDetail | Contact (Contact for public / scientific queries) | ||||
   | 0..1 | string | Unique id for inter-element referencing | |||||
   | N | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url | ||||
   | S | 0..1 | CodeableConcept | type de contact : Public ou Scientific URL: https://interop.esante.gouv.fr/ig/fhir/eclaire/StructureDefinition/eclaire-contact-type Binding: Type Contact Value Set (required) | ||||
   | ΣN | 0..1 | string | Name of an individual to contact | ||||
   | ΣCN | 0..* | ContactPoint | Contact details for individual or organization | ||||
  | TU | 0..* | RelatedArtifact | References and dependencies | ||||
  | ΣN | 0..* | CodeableConcept | Used to search for the study Binding: (unbound) (example): Words associated with the study that may be useful in discovery. | ||||
  | SΣ | 0..* | CodeableConcept | Pays de recrutement / Countries of Recruitment Binding: Jurisdiction ValueSet (extensible): Countries and regions within which this artifact is targeted for use. | ||||
  | S | 0..1 | markdown | Résumé de l'essai / Summary Results | ||||
  | SΣC | 0..* | Reference(Group) | Critères d'inclusion et d'exclusion / Inclusion & exclusion criteria | ||||
  | ΣCN | 0..1 | Period | When the study began and ended | ||||
  | SΣC | 0..1 | Reference(Organization) | Promoteur / primary Sponsor | ||||
  | ΣCN | 0..1 | Reference(Practitioner | PractitionerRole) | Researcher who oversees multiple aspects of the study | ||||
  | SΣC | 0..* | Reference(Location) | Lieux / Countries of Recruitment | ||||
  | ΣN | 0..1 | CodeableConcept | accrual-goal-met | closed-due-to-toxicity | closed-due-to-lack-of-study-progress | temporarily-closed-per-study-design Binding: ResearchStudyReasonStopped (example): Codes for why the study ended prematurely. | ||||
  | N | 0..* | Annotation | Comments made about the study | ||||
  | N | 0..* | BackboneElement | Defined path through the study for a subject | ||||
   | 0..1 | string | Unique id for inter-element referencing | |||||
   | N | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url | ||||
   | ?!ΣN | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ||||
   | N | 1..1 | string | Label for study arm | ||||
   | N | 0..1 | CodeableConcept | Categorization of study arm | ||||
   | N | 0..1 | string | Short explanation of study path | ||||
  | N | 0..* | BackboneElement | A goal for the study | ||||
   | 0..1 | string | Unique id for inter-element referencing | |||||
   | N | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url | ||||
   | ?!ΣN | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ||||
   | N | 0..1 | string | Label for the objective | ||||
   | N | 0..1 | CodeableConcept | primary | secondary | exploratory Binding: ResearchStudyObjectiveType (preferred): Codes for the kind of study objective. | ||||
 Documentation for this format Documentation for this format | ||||||||
Other representations of profile: CSV, Excel, Schematron
| Path | Conformance | ValueSet / Code | ||||
| ResearchStudy.language | preferred | CommonLanguages
| ||||
| ResearchStudy.identifier:idPrimary.use | required | Pattern: official | ||||
| ResearchStudy.identifier:idPrimary.type | extensible | Identifier Type Codes | ||||
| ResearchStudy.identifier:idSecondary.use | required | Pattern: secondary | ||||
| ResearchStudy.identifier:idSecondary.type | extensible | Identifier Type Codes | ||||
| ResearchStudy.status | required | ResearchStudyStatus | ||||
| ResearchStudy.primaryPurposeType | extensible | ResearchStudyPrimaryPurposeType | ||||
| ResearchStudy.phase | extensible | EclaireStudyPhaseVS | ||||
| ResearchStudy.category | example | |||||
| ResearchStudy.focus | example | |||||
| ResearchStudy.condition | example | Condition/Problem/DiagnosisCodes | ||||
| ResearchStudy.keyword | example | |||||
| ResearchStudy.location | extensible | Jurisdiction ValueSet | ||||
| ResearchStudy.reasonStopped | example | ResearchStudyReasonStopped | ||||
| ResearchStudy.objective.type | preferred | ResearchStudyObjectiveType |
| Id | Grade | Path(s) | Details | Requirements |
| cpt-2 | error | ResearchStudy.contact.telecom | A system is required if a value is provided. : value.empty() or system.exists() | |
| ele-1 | error | **ALL** elements | All FHIR elements must have a @value or children : hasValue() or (children().count() > id.count()) | |
| ext-1 | error | **ALL** elements | Must have either extensions or value[x], not both : extension.exists() != value.exists() | |
| per-1 | error | ResearchStudy.identifier:idPrimary.period, ResearchStudy.identifier:idSecondary.period, ResearchStudy.period | If present, start SHALL have a lower value than end : start.hasValue().not() or end.hasValue().not() or (start <= end) | |
| ref-1 | error | ResearchStudy.identifier:idPrimary.assigner, ResearchStudy.identifier:idSecondary.assigner, ResearchStudy.protocol, ResearchStudy.partOf, ResearchStudy.enrollment, ResearchStudy.sponsor, ResearchStudy.principalInvestigator, ResearchStudy.site | SHALL have a contained resource if a local reference is provided : reference.startsWith('#').not() or (reference.substring(1).trace('url') in %rootResource.contained.id.trace('ids')) |